This article was first released as Pharm Daily Premium Content on September 11, 2024, at 09:30.
‘
‘
‘
‘[Edaily Shin Min-joon Journalist Song Young-du] Hanmi Pharmaceutical (128940) is accelerating the development of future food, obesity treatment. Hanmi Pharmaceutical is set to launch the first long-lasting and personalized GLP-1 obesity treatment called Efpeglenatide, which applies proprietary technology platform technology by 2027.’
‘
‘
‘Hanmi Pharmaceutical is also developing a next-generation obesity treatment triple-action agent (GLP-1, glucose-dependent insulinotropic peptide (GIP), glucagon (GCG)) that minimizes muscle loss while expecting a 25% or more weight loss. Through various obesity treatments, Hanmi Pharmaceutical actively aims to capture the global obesity treatment market worth about 65 trillion won by 2028.’
‘
‘
‘
|
|
| Choi In-young, head of Hanmi Pharmaceutical’s R&D Center, presents at the 8th Edaily Global Pharma Bio Conference held at Harmony Hall, KG Tower on Tongil-ro, Jung-gu, Seoul on the 10th. (Photo = Edaily, Reporter No Jin-hwan) |
|
‘
‘Efpeglenatide to be released by 2027… Development of triple-action agent
‘
‘
‘Choi In-young, head of Hanmi Pharmaceutical’s Research and Development (R&D) Center, presented at the 8th Edaily Global Pharma Bio Conference held with the theme ‘K-Bio, asking for success strategies in the obesity treatment market’ at Harmony Hall, KG Tower in Jung-gu, Seoul on the 10th. Choi introduced Hanmi Pharmaceutical’s obesity treatment strategy called H.O.P (Hanmi Obesity Pipeline).’
‘
‘
‘H.O.P refers to Hanmi Pharmaceutical’s core project that sequentially introduces various ‘personalized therapies’ that can help in the pre-obesity treatment phase. The first treatment commenced in Hanmi Pharmaceutical’s H.O.P project is Efpeglenatide, which is considered a pioneering product. Efpeglenatide, a long-lasting GLP-1 obesity treatment, is being developed with Hanmi Pharmaceutical’s proprietary platform technology Labscuberi applied for the first time. Efpeglenatide is being developed as a personalized obesity treatment tailored to Koreans, reflecting their physique and weight, setting it apart from global competitive drugs.’
‘
‘
‘Last year, Hanmi Pharmaceutical received approval from the Ministry of Food and Drug Safety for the phase 3 clinical plan for Efpeglenatide. Hanmi Pharmaceutical started enrolling the first clinical trial participants for Efpeglenatide in December last year. The study is being conducted on 420 patients at six institutions, including Seoul National University Hospital.’
‘
‘
‘The expected end point for the phase 3 trial of Efpeglenatide is the second half of 2026. As a result, Efpeglenatide is expected to be commercially available by 2027. Efpeglenatide is being developed as a once-weekly injectable. In particular, Efpeglenatide is expected to alleviate drug scarcity issues in the obesity treatment market through economic pricing policies and stable supply due to Hanmi Pharmaceutical’s vertical integration from raw materials to manufacturing and sales.’
‘
‘
‘Even with a dosage of 6 milligrams (mg), Efpeglenatide is characterized by having the highest efficacy among GLP-1 peptide series drugs in terms of not only cardiovascular protection but also weight loss efficacy and safety.’
‘
‘
‘He said, “With a 6mg dose, Efpeglenatide reduced the major cardiovascular event (MACE) risk ratio to 0.65, lowering the risk by 35%” and added, “This is a remarkably promising figure as it is the highest level within the existing GLP-1 peptide series obesity treatment drugs.”
‘
‘
‘Choi, the center head, also expressed his expectations for the next-generation innovative obesity treatment HM15275 following Efpeglenatide. HM15275 is anticipated to achieve over 25% weight loss while minimizing muscle loss. HM15275 is specialized in obesity treatment by optimizing the actions of three receptors: GLP-1 peptide analogues, GIP, and glucagon. HM15275 is designed to have effects on various metabolic diseases as a secondary benefit. The current phase 1 clinical trial of HM15275 is progressing smoothly in the United States. HM15275 aims to enter phase 2 clinical trials next year and is expected to be commercially available around 2030.’
‘
‘
‘In particular, Choi pointed out that HM15275 is expected to become the top drug in the obesity treatment field thanks to its efficacy comparable to that of surgical weight loss therapy. While GLP-1 based drugs like Semaglutide and Tirzepatide have shown a weight loss effect of about 15-20% in obesity treatment trials, they do not reach the level of weight loss as in obesity metabolic surgery (25-30%), indicating an unmet medical need.’
‘
‘
‘HM15275 demonstrated superior quantitative and qualitative weight loss effects compared to existing treatments in obesity models with repeated administration. This efficacy clarified that the optimized triple pharmacological action of HM15275 leads to weight reduction through dietary regulation and increased energy metabolism.’
‘
‘
‘He said, “HM15275’s preclinical study results confirmed its potential for the highest weight loss and muscle loss reduction within its class,” and added, “The reduction in body fat was recorded at 66.5%, higher than 23.2% for HiGo-B and 38.1% for Zepbound. On the other hand, the muscle loss reduction was 7.7% for HM15275, outperforming Zepbound’s 10.4%.’
‘
‘
‘
|
|
| Concept of innovative obesity treatment. (Source=Hanmi Pharmaceutical) |
|
‘
‘Innovative obesity treatment, weight loss ↑, muscle ↑… AI design applied
‘
‘
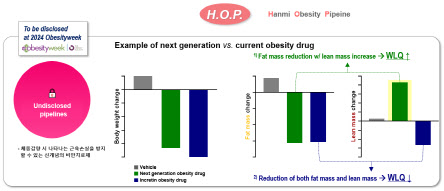
‘Choi explained Hanmi Pharmaceutical’s innovative obesity treatment. The innovative treatment has been praised for overcoming the limitations of existing treatments that accompany muscle loss (up to 40% of lost weight) during weight loss. The innovative therapy is designed to increase muscle mass during weight loss through a mechanism different from incretin-based therapies.’
‘
‘
‘The innovative obesity treatment, being peptide designed, has a competitive price compared to other obesity treatments. Additionally, the innovative obesity treatment sets itself apart by being designed with the help of artificial intelligence (AI). Hanmi Pharmaceutical plans to present the subjects and preclinical trial results of the innovative obesity treatment candidate at ObesityWeek, to be held in the USA in November.’
‘
‘
He said, “Existing treatments focus on preserving muscle that decreases along with weight loss. However, Hanmi Pharmaceutical is developing an innovative obesity treatment that can simultaneously increase muscle and regulate weight” adding, “In preclinical studies, we confirmed that compared to existing treatments, while fat reduction remains equivalent, muscle increase was observed.’
‘
‘
‘In addition to orally-administered obesity treatments, Hanmi Pharmaceutical is striving to develop products that can be applied to obesity prevention and management, such as digital therapeutics that can correct the lifestyle and medication adherence of obese patients.’
‘
‘
‘Regarding the convergence of digital medical devices and pharmaceuticals, Hanmi Pharmaceutical expects synergistic effects such as maximizing efficacy and improving safety by integrating Hanmi Pharmaceutical’s Korean tailored GLP-1 obesity treatment Efpeglenatide with digital medical devices.’
‘
‘
“He said, “We will do our best to ensure that the rapid and successful birth of the first new obesity drug developed from start to finish by a Korean pharmaceutical company with proprietary technology.”‘
‘
‘
‘According to the Korea Biotechnology Industry Association, the global obesity treatment market size reached $66.8 billion (about 90 trillion won) last year. The global obesity treatment market is expected to grow at an average annual rate of 48.4% until 2028, reaching a size of $480.3 billion (about 650 trillion won).’
‘
‘
Visited 4 times, 1 visit(s) today